The SynLink research project deals with the production of renewable fuels, such as methanol or long-chain hydrocarbons. This means considering the entire value chain from both a technical and an economic perspective – from production of synthesis gas using renewable electrical energy and carbon dioxide, and its subsequent chemocatalytic conversion into fuel, right through to practical application tests on cars and trucks – and demonstrating that a technology readiness level (TRL) of 5–6 has been achieved. Within the project, Fraunhofer CBP was tasked with carrying out long-term experiments for methanol production on a pilot scale with a TRL of 5–6.
SynLink – Synthetic e-fuels as key enabler for sector linking
Synthetic fuels from CO2 and regenerative energy
Power‑to‑X activities are moving more and more into the focus of research in connection with the energy revolution, as surplus electricity from renewable energy generation can also be used for the electrochemical production of basic chemicals. The use of regenerative energies is therefore no longer limited to the electricity sector, but is also increasingly expanding to the chemical sector. The basic idea here is the substitution of molecules previously obtained from crude oil and natural gas in the chemical and refinery industries by chemically identical molecules which are obtained from CO2, water and renewable energy.
Regenerative methanol to replace fossil-based
One such molecule is methanol, which is currently produced in Europe mainly by steam reforming from natural gas (production volume of 3000 t/d) and is used in a wide variety of applications, e.g. as a fuel additive, as a starting material in fuel cells or as a bulk chemical in the chemical industry. A conversion of the upstream feed streams in methanol synthesis to renewable molecules would avoid 1.53 metric tons of CO2 emissions per ton of methanol produced.
However, for an economic implementation of sustainable processes, significant cost reductions for process control are necessary, which are accompanied by corresponding optimization and scaling of the individual processes and its integration. There is also a need for research in order to increase the degree of technological maturity of technologies already developed for specific applications up to a size suitable for industrial use.
Value chain from regenerative syngas production to e-fuels application tests
In the "SynLink" project, the entire value chain from synthesis gas production using H2O, renewable electrical energy and CO2 (from air adsorption) to the chemo‑catalytic production of fuels to application tests of these fuels in cars and trucks is being investigated for the first time both technically and economically and demonstrated in the Fraunhofer Hydrogen Lab Leuna.
The core element of this project is the synthesis gas production by means of Co‑SOEC (co‑solid oxide electrolyser cell with 150 KW) in order to couple renewable electrical energies into the chemical value chain. The synthesis gas is converted via methanol synthesis with increased CO2 content or Fischer‑Tropsch synthesis. The raw product produced is further refined via various refining steps to produce e‑fuels.
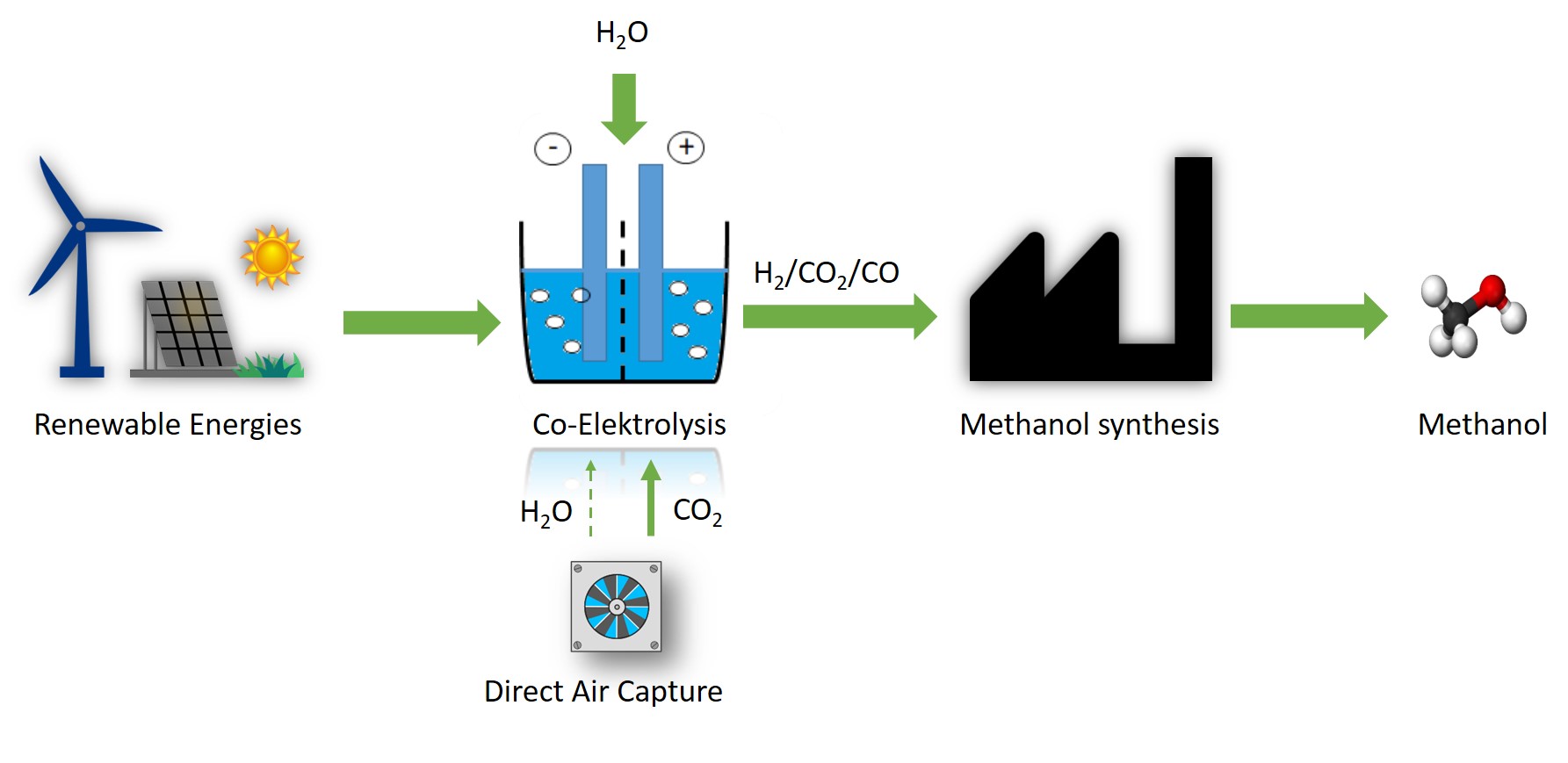
Synthesis of e-fuels for mobile applications

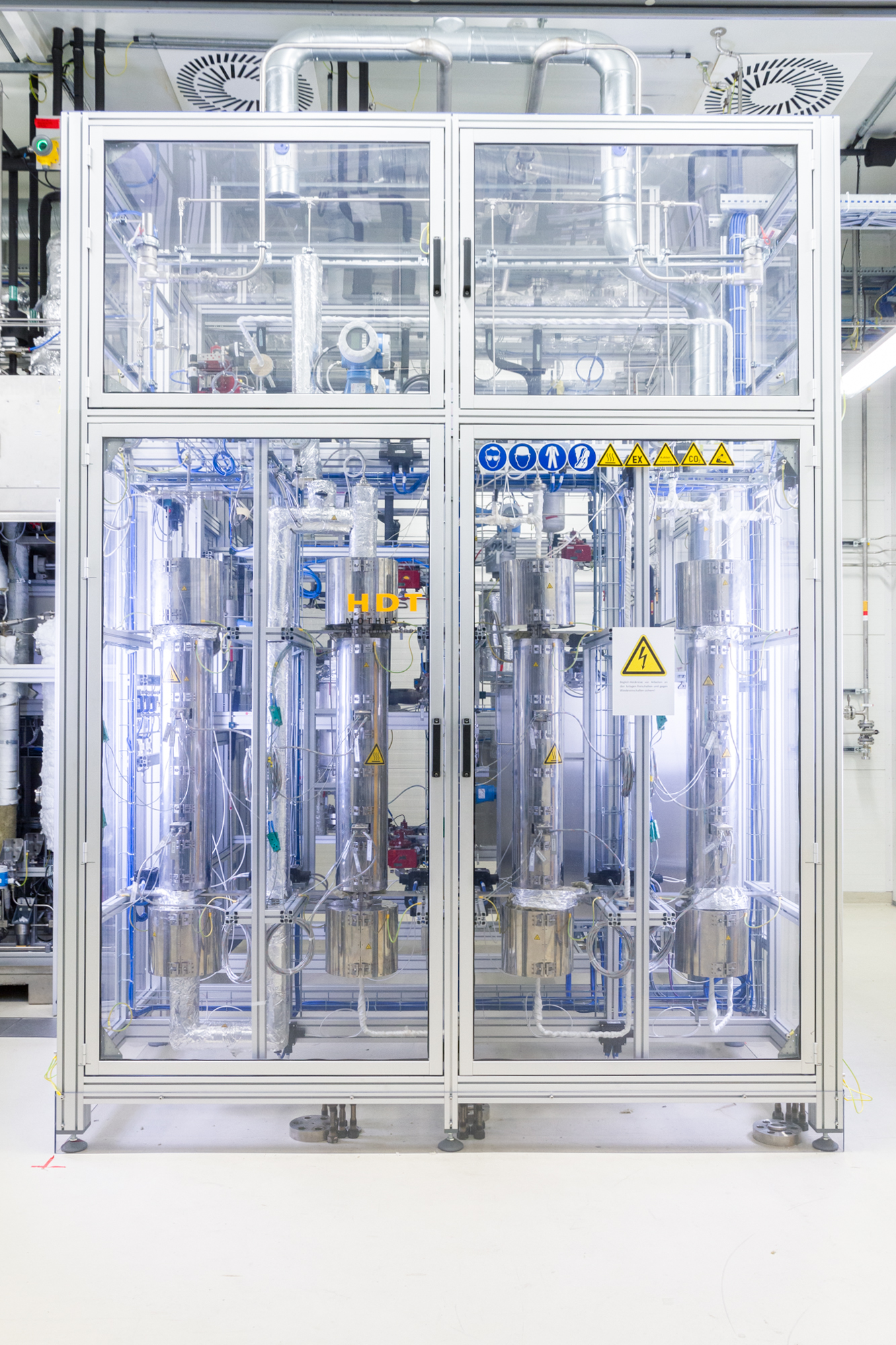

Within this project, Fraunhofer CBP is working on the further development of methanol synthesis from CO2‑rich synthesis gas, first on a laboratory scale (TRL 3) and further scaled‑up to a pilot plant (TRL 6). The integration of the Co‑SOEC process with the methanol pilot plant will be carried out and its technological feasibility tested. Sample quantities of up to 500 liters are to be made available for application testing.
Producing methanol from synthesis gas
Once a methanol plant had been engineered and set up on a laboratory scale at the CBP, the temperature (250–270°C), pressure (50–90 bar gauge) and gas hourly space velocity (GHSV; 8,000–30,000 h-1) were varied to determine the optimum process parameters while using an industrial catalyst.
During this process, the synthesis gas was converted into a methanol-water mixture using a plug flow reactor and an industrial catalyst. Any non-converted synthesis gas was separated by a separator and fed back to the reactant flow. As the process parameters were varied, their influence on the conversion process was examined, long-term tests were carried out in relation to catalyst stability and the results of the experiments were compared with the simulations that were being conducted in parallel with the process.
The results of the experiments demonstrated that the conversion rate could be increased by up to 44 percent by raising the pressure. By contrast, increasing the GHSV led to a reduction in the conversion rate. This can be explained by the shorter contact time with the catalyst. Within the investigated temperature range, the temperature was not found to have any influence on the conversion rate. The results from the simulations were comparable to those from the laboratory-scale experiments.
Piloting the methanol process
Once methanol synthesis had been established on a laboratory scale, a pilot plant was converted to carry out the methanol process on a pilot-plant scale and then put into operation. The operating point was scaled to the highest conversion rate and the plant was operated on a long-term basis with subsequent rectification of the methanol-water mixture. The conversion rate and purity of the methanol produced in the pilot plant were similar to those achieved in the laboratory experiment.
Application tests
For the subsequent practical application tests on the cars and trucks, the methanol produced from the synthesis gas was used as a fuel in both blended and pure form. Given that no standard has yet been drawn up for using methanol as a fuel, possible requirements for the product were defined on the basis of DIN 51625 “Automotive fuels – Ethanol fuel – Requirements and test methods”.
Methanol synthesis on behalf of customers
Following the successful completion of the project, Fraunhofer CBP now has the expertise required to carry out methanol synthesis on behalf of interested companies and organizations on both a laboratory and a pilot scale.
Project information
Project title
SynLink – Synthetic e-fuels as key enabler for sector linking
Project duration
January 2019 – September 2022
Project partners
- Sunfire GmbH, Dresden (Coordination)
- Climeworks Deutschland GmbH, Dresden
- DLR-Institut für Technische Thermodynamik, Stuttgart
- EDL Anlagenbau Gesellschaft mbH, Leipzig
- EIFER Europäisches Institut für Energieforschung, Karlsruhe
- Fraunhofer IKTS, Dresden
- Fraunhofer IMWS, Halle
- Fraunhofer ICT, Pfinztal
- Fraunhofer ISE, Freiburg
- Total Research & Technology Feluy, Feluy, Belgium
- Airbus Defence and Space GmbH, Munich
- Analytik-Service Gesellschaft mbH, Neusäß
- Daimler AG, Stuttgart
- Ford Werke GmbH, Köln
- KIT IFKM, Karlsruhe
- Mahle International GmBH, Stuttgart
- ISUZU MOTORS Germany GmbH, Ginsheim-Gustavsburg
- Airbus Defence and Space GmbH, Taufkirchen
Funding
We would like to thank the German Federal Ministry of Economic Affairs and Climate Action (BMWK) for funding the project "SynLink – Synthetic e-Fuels as Key Enabler for Sector Linking", promotional reference 03EIV03E.

 Fraunhofer Center for Chemical-Biotechnological Processes CBP
Fraunhofer Center for Chemical-Biotechnological Processes CBP