Sterilization with gamma rays

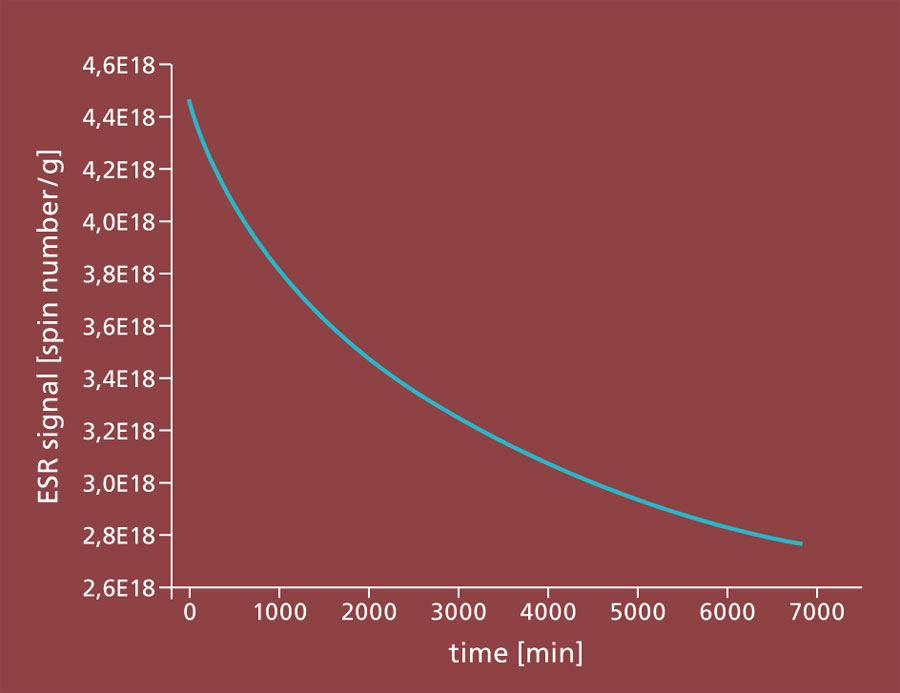
Gamma sterilization is increasingly being used to preserve food or sterilize heat-sensitive pharmaceutical products. For pharmaceuticals and medical devices, the World Health Organization (WHO) specifically recommends this sterilization, in which products are irradiated with high-energy gamma rays from a cobalt-60 beam source (Figure 1). As a result of the irradiation, the genomes of germs and pathogenic microorganisms are destroyed and the organisms are killed, significantly extending the shelf life of the products. Compared to sterilization with ethylene oxide or steam sterilization, the treatment is very gentle. Another advantage of treatment with gamma rays is that products can be sterilized or sterilized in their packaging - without any significant increase in temperature or the use of chemicals.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB