Ionic liquids (IL) – designer fluids for customized properties
Ionic liquids (IL) are salt-like compounds that are liquid at temperatures below 100°C. The wide range of possibilities for combining different anions and cations offers the potential to tailor their properties to specific applications.
Different gas absorption for selective separation
It has been shown, for example, that ionic liquids absorb gases to different extents. While N2 and H2 are only absorbed to a small extent, stronger absorption is observed for other gases such as CO2. This difference can be used for the selective separation of individual gases from gas mixtures. For example, it is conceivable to use ionic liquids instead of the usual amine solutions in absorbers for the treatment of combustion gases from coal and gas-fired power plants.
Broad investigation of ionic liquids for gas absorption
In order to assess the suitability of ionic liquids for gas absorption, we tested a broad combination spectrum of different anions and cations to investigate the structure-property relationship. The pressure decay method was used to determine the gas absorption capacity for carbon dioxide, nitrogen, methane and carbon monoxide in different ionic liquids.
High solubility of CO2 and CH4
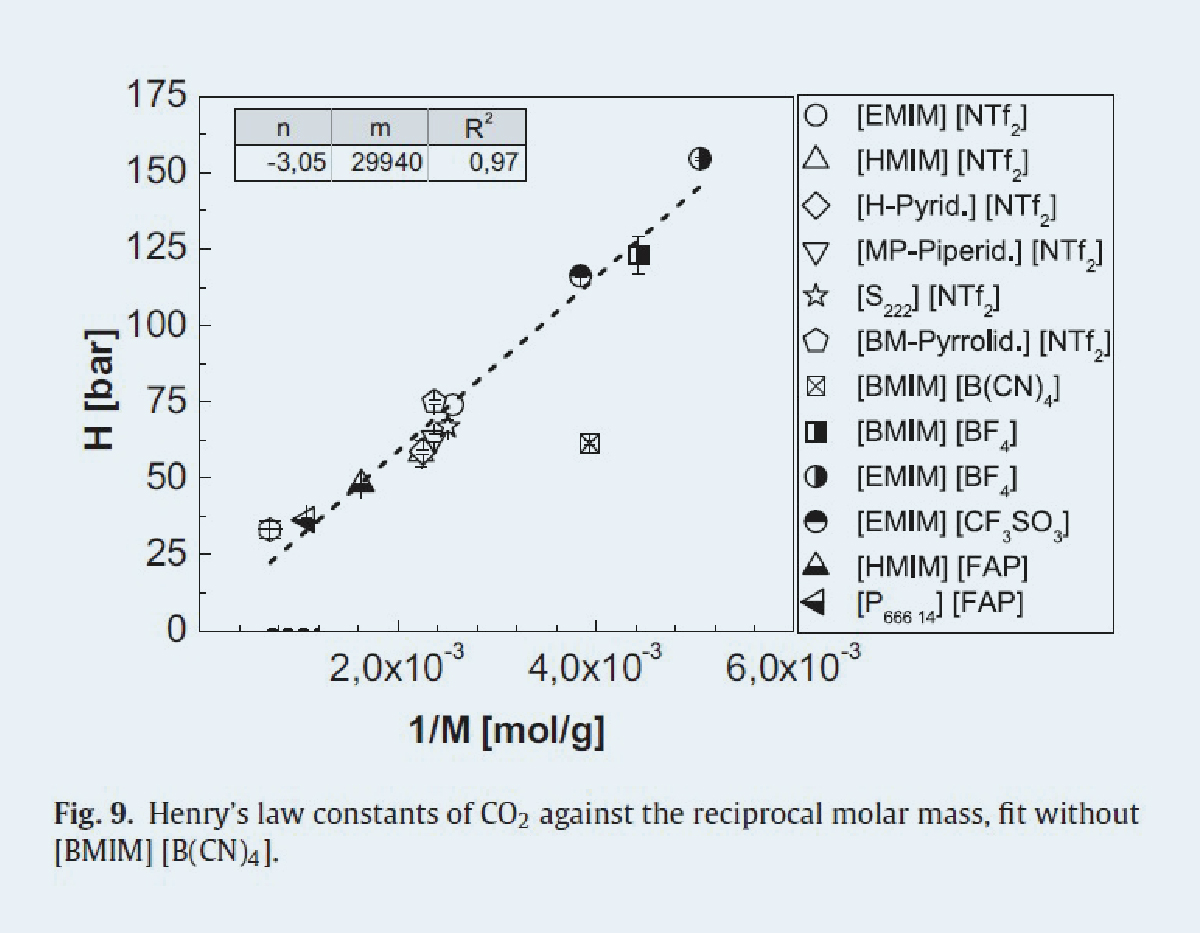
We were able to demonstrate the highest gas solubility in all ionic liquids for carbon dioxide, followed by methane. Nitrogen and carbon monoxide which dissolve in only very small amounts in the ionic liquids studied. From the data obtained, a linear dependence between the reciprocal molar mass and Henry's constant, a measure of gas absorption capacity, can be derived for the physisorption of CO2, N2 and CH4 in ionic liquids [1]. Thereby, a small Henry constant stands for a high absorption capacity per mole of ionic liquid. Figure 1 shows this model using CO2 at 60 °C as an example.
High loading capacity of ionic liquids containing imidazolium for CO2
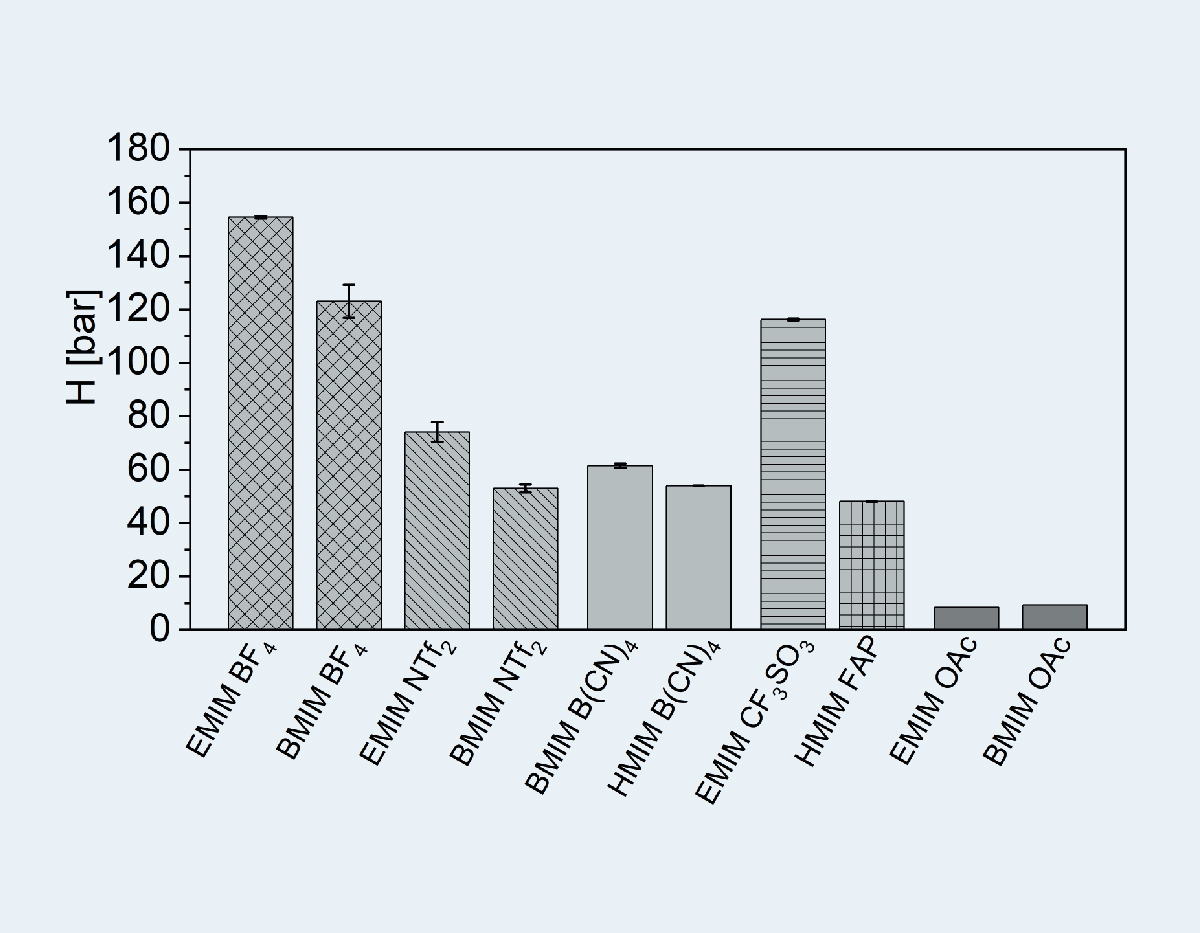
In addition, a chemical interaction between ionic liquids containing imidazolium with a basic anion and carbon dioxide has been demonstrated. This leads to significantly higher loading capacities [2]. The basicity of the anion has a significant influence on the absorption capacity. Small pKB values tend to lead to small Henry constants and, at the same time, to high absorption rates in the ionic liquids (up to 75 g CO2 per kg ionic liquid). The CO2 absorption capacity of imidazolium-containing ionic liquids with basic anion is thus of a similar order of magnitude to that of the currently established amine solutions for gas scrubbing. Unlike amines, however, ionic liquids are not volatile due to their low vapor pressure.
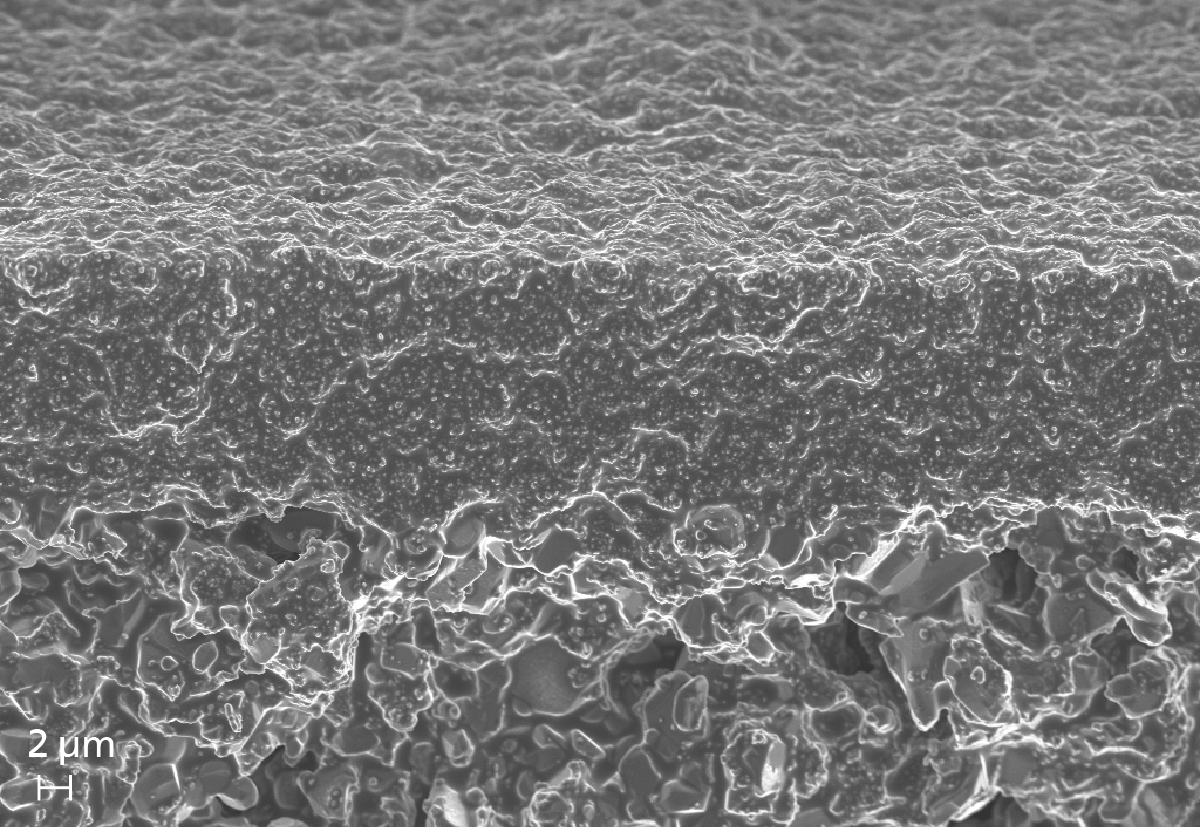
These results show the great potential of ionic liquids for gas treatment through the selective uptake of carbon dioxide. There is also the possibility of immobilizing ionic liquids in porous support structures via capillary forces and subsequently using them as liquid membranes, which are stable over the long term due to the low vapor pressure of the ionic liquids.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB