Many questions in modern biotechnology are to be answered by experiments involving the use of recombinant cloned transcripts. Hereby, the supply of specific proteins with custom-made linker or labeling functions, so-called functional tags, is getting more and more important. This is valid in particular for the interdisciplinary research field of interfacial- and nanotechnology and molecular biology, in which such molecular tools are used.
Manufacture of recombinant proteins with functional tags
Custom-made proteins for applications in proteomics

Fig. 1: Production of fusion proteins with functional tags: EGFP (enhanced green fluorescent protein) in E. coli.
Left: EGFP construct in E. coli pellets induced via IPTG. Right: control, non-induced EGFP construct.
By the methods of genetic engineering it is possible to insert almost any well-known gene from any organism in a suitable expression system so that the protein can be manufactured and purified. The Fraunhofer IGB offers the design and the production of such custom-made, biologically active proteins in the milligram scale as a research service. We are happy to provide you an offer according to your specifications (modification, expression system, degree of purity, scale, intended purpose).
Possible applications of fusion proteins:
- Protein/protein interactions: Identification of interacting proteins (co-immunoprecipitation, two hybrid assays)
- Representation of protein microarrays
- Production of antibodies against native proteins
- Use of labeled proteins (fusion proteins) for protein localization in mammalian cells / overexpression
- Use of recombinant peptides and proteins in the clinical therapy
Cloning and expression strategy
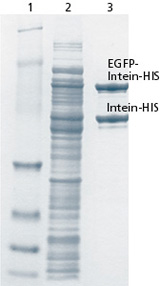
Fig. 2: SDS PAGE for controlling the protein purification process.
Lane 1: Protein marker (size),
Lane 2: Total protein after cell disruption (French Press), SDS-PAGE and Coomassie staining.
Lane 3: Purified target proteins (EGFP-Intein-HIS and Intein-HIS).
- Strategy planning
- Data base search
- Design of construct (fusion protein, selection of a suitable tag)
- Selection of a suitable expression system (eucaryotic, procaryotic)
- Specific primer design (loops, hairpins)
- PCR amplification of the gene of interest
- Enzymatic manipulations
- Ligation / transformation into the appropriate cloning system
- Screening for mutants
- Evaluation of cloning success by sequencing
- Expression of the target protein
- Induction optimization
- Cell disruption
- Different purification steps
- Renaturation
- Determination of activity
- Immobilization
- Measurements via MALDI TOF MS
References
For example we have constructed the following fusion proteins and provided them as native proteins in the milligram scale:
- The cytokine macrophage migration inhibitory factor (MIF) with N-terminal streptactin tag (MIF-StrepTag)
- Enhanced green fluorescent protein / Intein /Chitin binding protein fusion protein (EGFP-Intein-CBD)
- Enhanced green fluorescent protein / Intein /10 x Histidine-tag fusion protein (EGFP-Intein-HIS)
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB