Patent protection for the first biopharmaceuticals is expiring in the next few years. The manufacture of so-called biosimilars or biogenerica is thus increasingly gaining in importance. A considerable proportion of the production costs of a pharmaceutical protein is the result of high purification costs. Advances in the field of analytical characterization of products have challenged the dogma that "the process determines the product". These advances open new paths to the manufacture of safe and, due to alternative cleaning processes, inexpensive biopharmaceuticals which then become globally available to more patients.
Improved manufacturing process for coagulation factor VII
Blood coagulation factor VIIa
As an example the Fraunhofer IGB has developed an improved recombinant expression process as well as an improved manufacturing and conditioning method for coagulation factor VIIa (FVIIa), an essential factor for the blood coagulation cascade. Factor VIIa is a glyco-protein with N and O glycosylations and contains several gamma-carboxy-glutamic acid residues in the N-terminal area as a further modification. Thus, it is characterized by a charge heterogeneity which is shown by isoelectric focusing. The original glutamic acid residues are modified post-translationally in a complex, vitamin K-dependant process. These modifications bind bivalent cations. From a physiological point of view these are the calcium ions.
Cost-intensive conditioning due to complex media
The Novo approved product is expressed and exported with genetically engineered animal cells, in media which contain fetal calf serum (FCS). The complex composition of the media in which the target protein is only contained as a minor component makes the downstream processing (DSP) difficult. Thus, all FCS components in the medium and all proteins from lysed cells are to be separated by means of multi-stage separating processes. Affinity chromatography processes reveal the highest selectivity. The process used here is an immuno-affinity chromatography whereby immobilized antibodies (AB), which are directed against the target protein, bind the target protein with a high selectivity. The following cleavage of the AB-protein bond and thus the elution of the target protein must occur under conditions which damage neither the target protein nor the immobilized antibody. This means costly development of a suitable proprietary monoclonal antibody for the DSP which needs to be expressed in a cell line, immobilized and separated from the target protein as the decomposition of the substrate cannot safely be avoided.
Advantageous purification for defined media
Advances in the understanding of metabolism and physiology of animal cells facilitate novel media for cell cultures which do not need complex and barely defined components such as FCS. This ensures an improved product purity and safety. In addition, defined media provide chances for a simpler and less costly DSP for which only commercially available and comparatively cheap standard separation media such as ion exchange chromatography (IEX), hydrophobic interaction chromatography (HIC) and gel filtration are used. The relevant documentation for these separation media already exists for approval by the authorities, i.e. there is no need for them to be created and submitted by the manufacturer. The Fraunhofer IGB used a special serum-free and low-protein medium for the recombinant manufacture of factor VII into which factor VII is secreted as a proenzyme.
Results: purification with high selectivity
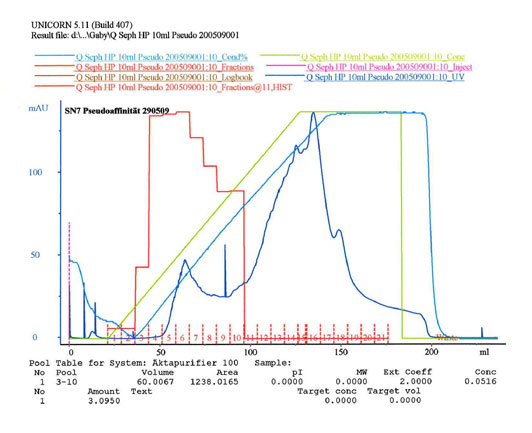
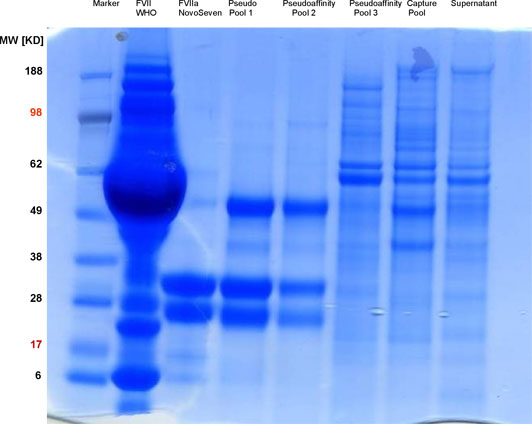
Anion exchange materials have been used for the purification of FVII and its activation as FVIIa. A high degree of selectivity has been achieved through the special properties of the target protein whose net charge is influenced via calcium concentration. When the concentrations of free calcium ions are low, the gamma-carboxy-glutamic acid residues are present with a double negative charge. These charges are neutralized with bound calcium. The net charge of the protein molecules impacts both the binding and the elution behavior on the ion exchanger. With a combination of two separation steps on the anion exchange materials on which the concentration of free calcium ions was modified; it was thus possible to achieve a highly selective purification. Such highly selective separation processes with standard separating media are also called "pseudo-affinity chromatography".
As factor VII can, under suitable conditions, be transferred into the target factor VIIa on positively charged boundary layers and thus also on anion exchange materials, a purification scheme which only utilizes anion exchangers and membrane processes will be used making the production of this biogeneric product cost-efficient.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB