The broad use of VLPs is currently limited by the lack of standardized and efficient manufacturing processes for bringing different VLPs with specific loads to different sites of action, similar to a modular system. In addition, the purification steps in particle production must be developed specifically for each protein or VLP. This empirical and individually adapted approach is time and cost intensive and thus a challenge for industrial production.
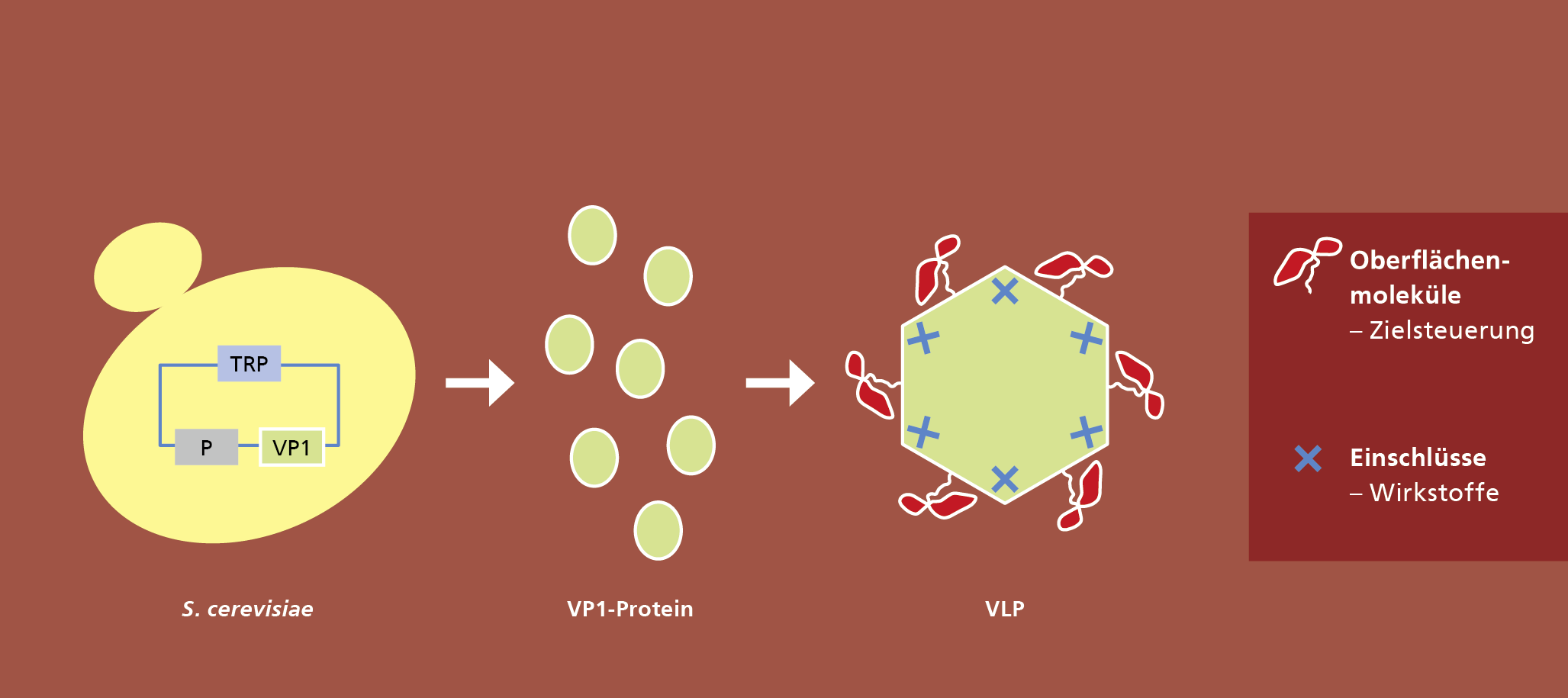
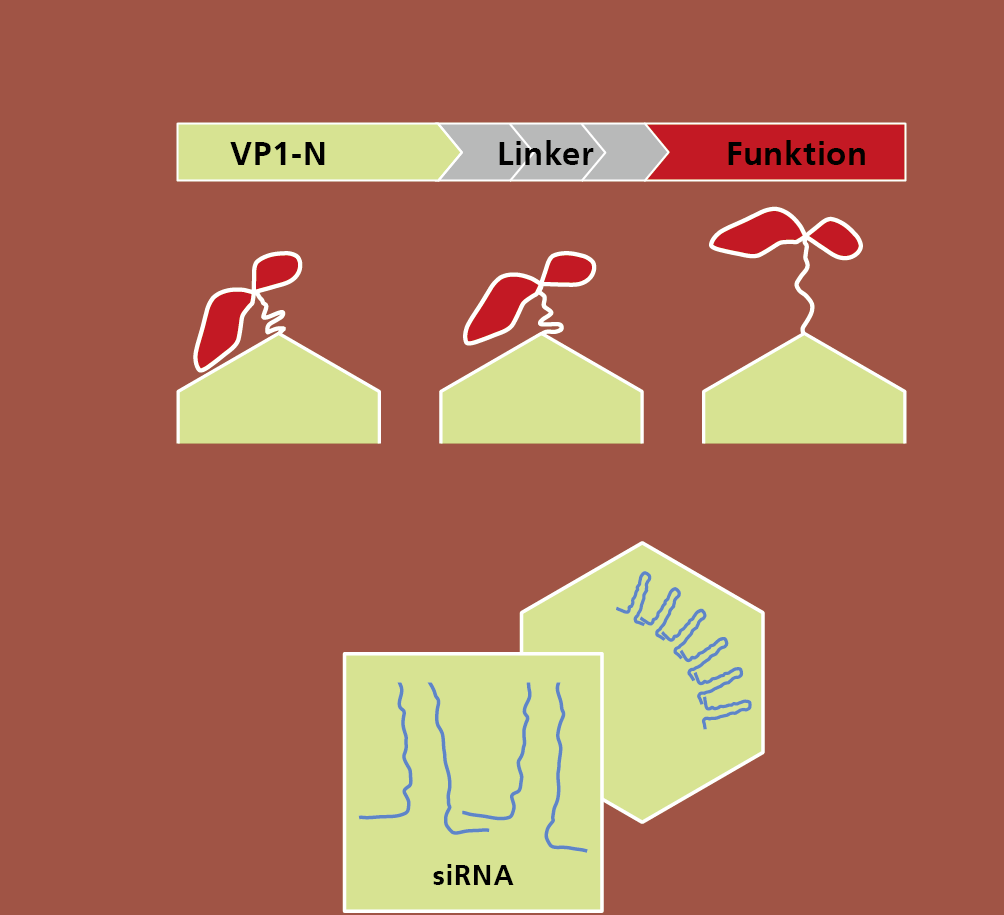
In a new project at Fraunhofer IGB we want to develop a kind of modular system as a platform technology for the production of VLPs. A basic module with an inner capsule structure will be genetically combined with a functional, variable and complex protein surface, which will be used either for target control of VLPs (drug delivery) or for vaccine development.
Antibody fragments or antigens, for example, can be used for multivalent functionalisation of the surface. Since the basic module always remains unchanged and only the protein surface is specifically designed, the production of VLPs can be standardised in comparison to current systems and thus be reproducible and cost-effective.
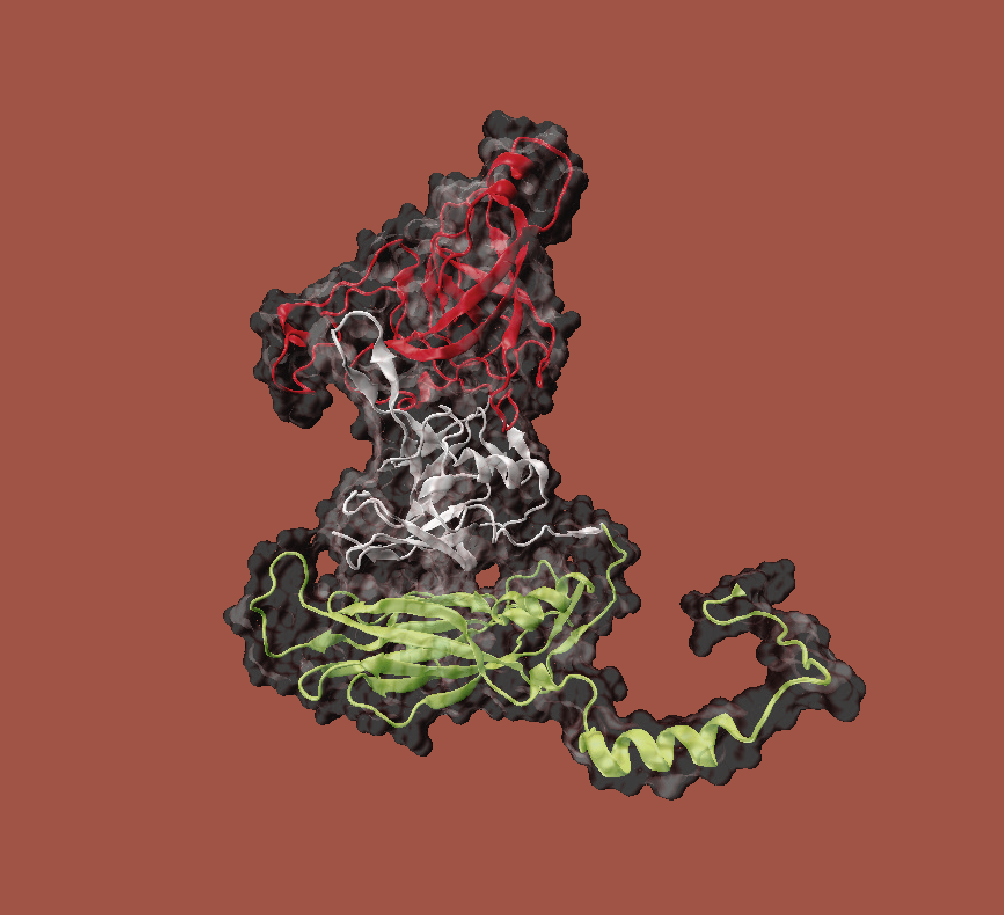
Envelope free viruses of the family Caliciviridae are used as a basis. For the synthesis of VLPs in baker's yeast (Saccharomyces cerevisiae) plasmids were developed which code for variants of the viral proteins. Baker's yeast is particularly well suited for the production of VLPs, as proteins for pharmaceutical applications can be produced in this organism with few side effects and at low cost.
A basic VLP structure has already been successfully produced and characterised in this way. This provides a system for the expression and isolation of native caliciviral VLPs so that downstream processing can be established. The aim is to establish an efficient and cost-effective process on the basis of these VLPs, which will enable the production of such biocontainers in large quantities and with high purity and homogeneity.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB