Therapeutic proteins continuously acquire higher shares of the pharmaceuticals market. Monoclonal antibodies, for example, show double-digit growth rates and also proteins which have been known for a longer period of time, such as interferon-β (IFN-β) exhibit an annual turnover of three billion US dollars. Treatment with IFN-β is one of the current basic therapies for the most frequent form of multiple sclerosis (MS), relapsing-remitting MS. The costs for IFN-β treatment in Germany amount to 18,000 euros per patient and year with the consequence that the majority of the 2.5 million MS patients worldwide cannot be adequately treated with IFN-β. In this context, the required quantities of active substances at 45 mg per patient and year for IFN-β-1b are very small.
Extraction and purification process for interferon-beta-1b
Two forms of IFN-beta
There are currently two forms of IFN-β on the market. The recombinant form prepared in animal cells (IFN-β-1a) corresponds to the human form in amino acid sequence and glycosylation. In contrast, the commercially available recombinant variant expressed in E. coli is not glycosylated and differs from the natural human form at its N-terminus (methionine is cleaved) and in that at position 17 cysteine has been replaced by serine. The form prepared in E. coli is termed IFN-β-1b. The patent protection of the two forms is running out. As a result, the opportunity of making IFN-b available as biosimilar to a broader group of patients more reasonably and in many parts of the world for the first time ever has arisen.
Criteria for an optimized production process
The objective of a current project at Fraunhofer IGB is the development of a procedure for preparing IFN-β-1b on an industrial scale. In this context, the price, the safety of the products, the stability of the processes (ruggedness), and the scalability are of central importance. A high expression rate, low costs for media and fermentation, as well as simple and stable procedures for isolation and purification (downstream processing) are decisive for the economic success and for safe patient care.
Cloning and expression
Scientists at Fraunhofer IGB have been able to establish a highly productive clone, which bears an IFN-β-1b gene sequence adapted to E. coli. This allows the expression of the desired IFN-β-1b proteins within the cells as inclusion bodies. As a result of high-cell-density fermentation, a stable and high expression rate of at least 20 percent IFN-β-1b in the total cell protein is achieved with this clone.
Scalable purification processes

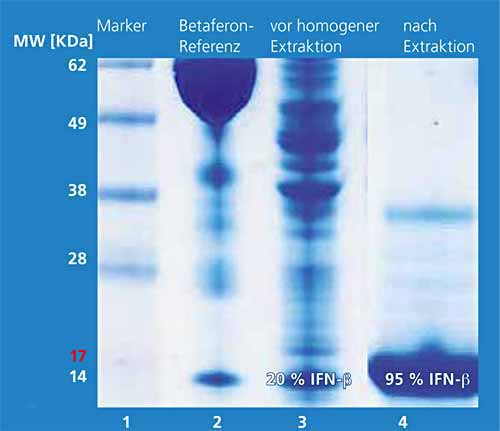
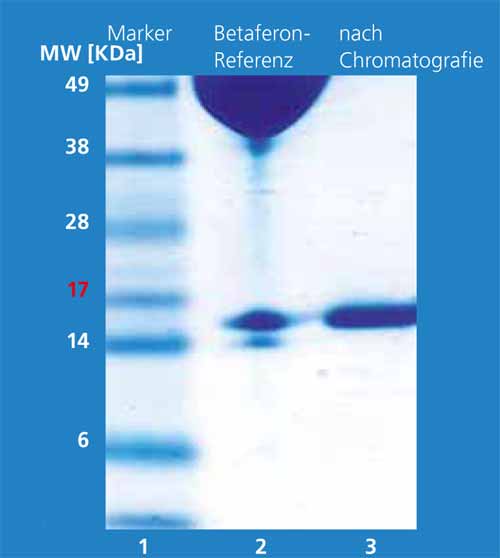
Isolation, purification, and solubilization of the IFN-β proteins from inclusion bodies have been a technically complex process up to now. The objective was to work out a technically simple and scalable downstream process [DFW1] for IFN-β-1b. For this, the bacterial cells are disrupted immediately after fermentation with a combined enzymatic-mechanical process. From this cell homogenate, the target protein is obtained with high yield and selectivity in only one extraction step with 2-butanol. The hydrophobic interferon-b moves upward into the upper organic phase (2-butanol) with the aid of surfactants. Bacterial proteins are found concentrated as a solid layer[DFW2] between the lower aqueous and upper organic phase. The lower aqueous phase contains the hydrophilic substances of the cells such as salts and DNA/RNA. Figure 1 shows the three phases after a phase separation forced by centrifugation. Figure 2 shows the protein pattern by means of SDS-PAGE before the extraction step (lane 3) and in the organic phase (lane 4). The target protein is present with a fraction of 20 percent before the extraction step; after extraction it has a purity of 95 percent. The following chromatographic steps increase the purity even further (Fig. 3).
Perspective
With the technically simple and scalable purification process for IFN-β-1b established at the Fraunhofer IGB, the majority of the bacterial proteins are separated directly in the first purification step. We can thus avoid the laborious isolation, purification and solubilization of the inclusion bodies, which result in high losses. This process supplies IFN-β-1b in high purity, which can be reasonably manufactured on an industrial scale.
Project partners
- Cinnagen, Teheran, Iran
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB