In traditional sterilization methods, microorganisms are inactivated through various physical or chemical agents such as heat, radiation or the influence of gases. The choice of the sterilization method depends on the material composition, the objective of the treatment and the range of application. With thermolabile materials such as medical products or equipment in medical en-gineering, thermal sterilization processes are not suitable. Gas sterilization procedures using explosive, toxic or carcinogenic gases are not optimal as well because they require high safety standards. Sensitive materials can be even degraded or irreversibly damaged by reactive chemical compounds during the gas sterilization process. Plasmas are successfully used in technical etch-ing and cleaning processes. The reactive particles of the plasma, thus making the use of water- or air-charged che-micals superfluous in these steps of the treatment. As a result of these mechanisms of interaction, plasmas are also suitable for sterilization methods.
Plasma sterilization for thermolabile materials
Plasma sterilization – an alternative?
The sterilizing action of low-temperature plasmas – inactivating microbial cells – offers a material-friendly alternative. In addition, the reactive particles in the plasma (excited molecules, radicals, ions accelerated in the electric field, and photons) remove organic contaminations such as cell residues. The sterilizing gas mixtures are in fact produced directly in the plasma and no costly disposal of xyz is necessary.
Process development

At the Fraunhofer IGB an interdisciplinary team of plasma physists and biologists is working on the development of plasma sterilization processes towards the industrial application level. Various operating conditions and gas mixtures have been tested with respective diag-nostic processes in a plasma reactor de-veloped at Fraunhofer IGB (Fig. 1). Using microbiological evaluation methods, where polymeric materials were deliberately contaminated with microorganisms, we were able to investigate the mechanisms which contribute to inactivation and to determine the boundary conditions for an effective sterilization effect.
Plasma inactivates microorganisms
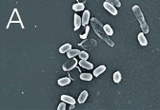
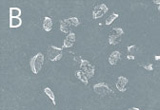
We were able to show, that various types of highly resistant bacterial endospores are not able to survive in the plasma even within relatively short treatment times and that the temperature increases only very slightly. Even with a relatively low power the initial cell number in standardized samples with a defined number of spores could be reduced by a factor of greater than 107 after just three minutes (Fig. 2). Scanning electron microscope images show a significant degradation of the cell substance after such a plasma treatment (Fig. 2).
Plasma degrades pyrogens
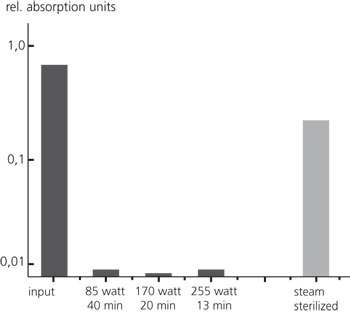
On one hand medicinal products and medical devices such as implant materials, surgery instruments, endoscopes and catheters have to be sterile, i.e. free of living bacteria. Furthermore, it has to ensured that they are free of pyrogens, fever-inducing residues of fungi or bacteria which can cause blood poisoning if they enter a patient’s bloodstream. Be-side the sterilizing effect, an appropriate plasma treatment leads to degradation of cell residues and components. Using suitable methods for the detection of pyrogens we could verify that the inactivation of the microbial cells is accompanied by a significant depyrogenization of the materials (Fig. 3).
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB