Lateral flow assays (LFA) offer a rapid chromatographic test method that can be used anywhere and without the need for special equipment. Fraunhofer IGB is investigating the use of nucleic acid-based detection methods in LFA format in order to provide a fast point‑of‑care test for infectious agents.
Development of a lateral flow assay for the nucleic acid-based molecular detection of pathogens
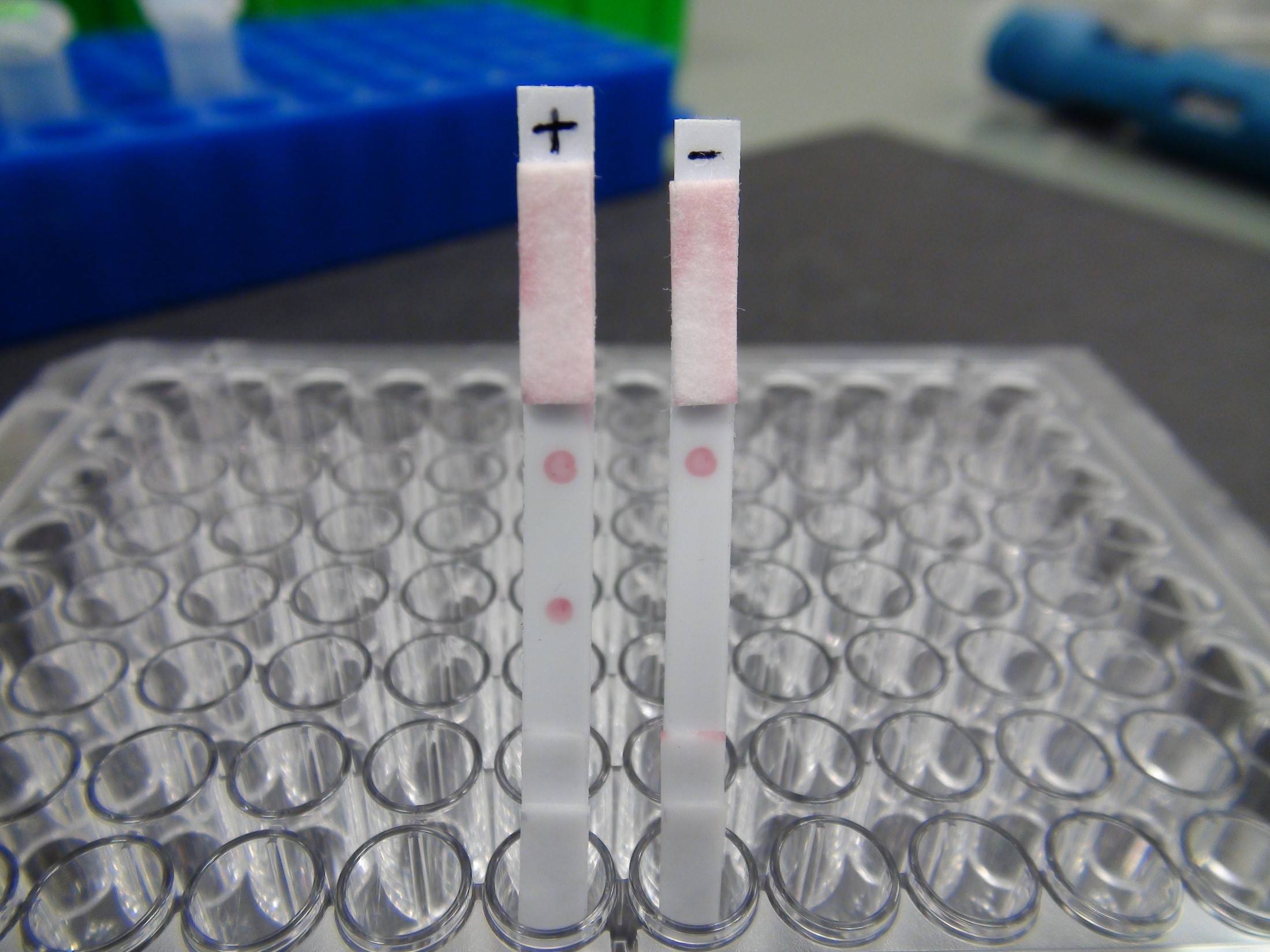
A lateral flow assay (LFA) is a rapid chromatographic test method. It does not require infrastructure or equipment and is therefore suitable for use in many applications as a point‑of‑care test (POCT). Chemical or immunological LFAs exist for some time, the best‑known being the pregnancy test. However, there is still need for development of nucleic acid-based detection methods in LFAs (NALFAs), which could pave the way for the molecular detection of pathogens at the point‑of‑care. NALFAs would enable the rapid implementation of specific therapeutic measures and inhibit further spread of pathogen.
In cooperation with the High‑Performance Center for Mass Personalization, researchers in the innovation field of Virus‑based Technologies have now developed a NALFA to detect herpes simplex virus 1 (HSV‑1) as proof of concept. To this end, the genomic DNA of the virus was specifically amplified by means of a polymerase chain reaction and modified for detection in an LFA. Here, modification of the genome target sequences and their hybridization with the immobilized and complementary capture DNA generates a color signal that indicates the presence of the specific pathogen (Fig. 1).
Based on these results, further developments are conceivable, including isothermal amplification of the pathogen genome as well as the parallel detection of several pathogens on one strip. The aim is to create a fast, device‑independent POCT for the detection of pathogens, which can be used in regions with poor medical infrastructure.
Further development for SARS-CoV-2 for the diagnosis of Covid-19
Thus, the test is also suitable for the rapid and reliable diagnosis of the novel coronavirus SARS-CoV-2. Such a rapid test would help to expand the diagnostic capacities and thus obtain more reliable data on the spread of Covid-19.
Project information
Project title
Development of a lateral flow assay for the nucleic acid-based molecular detection of pathogens
Project duration
January 2019 – December 2019
Project funding
High‑Performance Center for Mass Personalization
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB