A novel therapeutic advancement in the search for heart muscle progenitor cells – hope for heart attack patients
Breakthrough in heart research: The research team from Professor Katja Schenke-Layland of the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart has discovered cell surface markers that enable the identification and isolation of living functional cardiovascular progenitor cells (CPCs). For the first time, therapeutically relevant CPCs can be derived from induced-pluripotent stem cells (iPS) cells. CPCs, which are typically only found in fetal development, can become all of the different cell types of the heart and can integrate into heart muscle tissue after injection.
An estimated 17 million people die from cardiovascular disease each year. Although mortality rates are declining, heart attacks are still among the most frequent causes of death in the developed world. Often, the cause of a heart attack is the closure of a coronary artery that supplies blood to the heart, which kills heart muscle cells. Cardiomyocytes, which are the heart muscle cells responsible for the contraction of the heart, are not able to regenerate after a heart attack. The massive loss of cells and tissue, and the highly restricted regeneration capacity of the adult heart, lead to an impaired blood supply throughout the body that drastically affects a patient’s quality of life. To restore the heart’s function after a major heart attack, clinicians require functionally mature cardiomyocytes that perform like the native cells in the adult heart to replace the cells that were killed.
The production of such functional cardiomyocytes from well-defined cardiovascular progenitor cells (CPCs) is the focus of the research team led by Prof. Dr. Katja Schenke-Layland from the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart and her colleagues, Dr. Ali Nsair of the University of California Los Angeles (UCLA) and Prof. Dr. Robb MacLellan of the University of Washington in Seattle, who have now succeeded in identifying such cells in a mouse model. The work, which could revolutionize the treatment of heart disease, was recently published in the journal PLoS ONE (PLoS ONE7 (10):e45603, doi:10.1371/journal.pone.0045603).
Development of heart muscle cells from precursor cells
Myocardial cells – as well as endothelial cells and smooth muscle cells – develop from CPCs during the embryonic development of humans and other animals. There has been a significant amount of research effort towards discovering a path for the clinical application of these cells in patients. The reason for the lack of success is that the markers that help to identify CPCs, such as Islet1 or Nkx2.5, are located in the nucleus of the cells. The use of these cell markers modifies the cells rendering them therapeutically unusable, making the identification of safe cell-surface markers essential.
Surface markers identified for cardiovascular progenitor cells
On this task, the research team of Professor Katja Schenke-Layland from the Fraunhofer IGB in Stuttgart, Prof. Dr. Robb MacLellan and Dr. Ali Nsair of the University of California Los Angeles (UCLA), where Schenke-Layland previously worked before returning to Germany to join the Fraunhofer-Gesellschaft’s Attract Program, focused their research. With success: They were able to identify two markers, the receptors Flt1 (VEGFR1) and Flt4 (VEGFR3), on the surface of CPCs with which these cells can be clearly identified while fully preserving their biological function. This discovery allows scientists to isolate clinically relevant cardiovascular progenitor cells that can be functionally matured.
In the search for surface markers, the researchers investigated the cardiovascular progenitor cells using microarray gene expression profiling. These studies show exactly which genes are active at a specific point in time. The resulting data from this analysis were compared to the sequencing data from existing databases of already known as cell markers.
From induced-pluripotent stem cells, cardiovascular progenitor cells are developed
Encouraged by the success of being able to identify and isolate living CPCs, the researchers sought out to derive the cells from induced-pluripotent stem (iPS) cells. For this purpose, they used a method for which the Japanese scientist Shinya Yamanaka was recently awarded the 2012 Nobel Prize for Medicine. This work, published just six years ago, demonstrated that only four proteins are responsible for the embryonic state of cells (Takahashi K, Yamanaka S. Cell 2006, 126 (4): 663 -676). He brought those four genes into differentiated – mature and specialized – cells, which then returned them back to an embryonic state. From these cells, which he called iPS cells, scientists can develop all cells of the body, such as liver cells, nerve cells or heart muscle cells.
In their study, the researchers used cells from a mouse strain in which the cells are labeled with a visible green fluorescent protein (GFP) that can be identified with a fluorescence microscope. The cells from these mice were then reprogrammed with the same four genes discovered by Yamanka, resulting in iPS cells that could be easily identified.
In a next step, the researchers cultured the GFP-labeled iPS cells in the laboratory under different conditions with cell-influencing solutions such as growth factors. "Using our newly established cell surface markers, we could detect and isolate the Flt1 and Flt4 positive CPCs in culture," says Schenke-Layland. "When we cultured the isolated mouse CPCs then in vitro, they actually developed – as well as the embryonic stem cell-derived progenitor cells – into endothelial cells, smooth muscle cells and more interestingly into functional heart muscle cells."
iPS cell-derived CPCs integrate into the living mouse heart
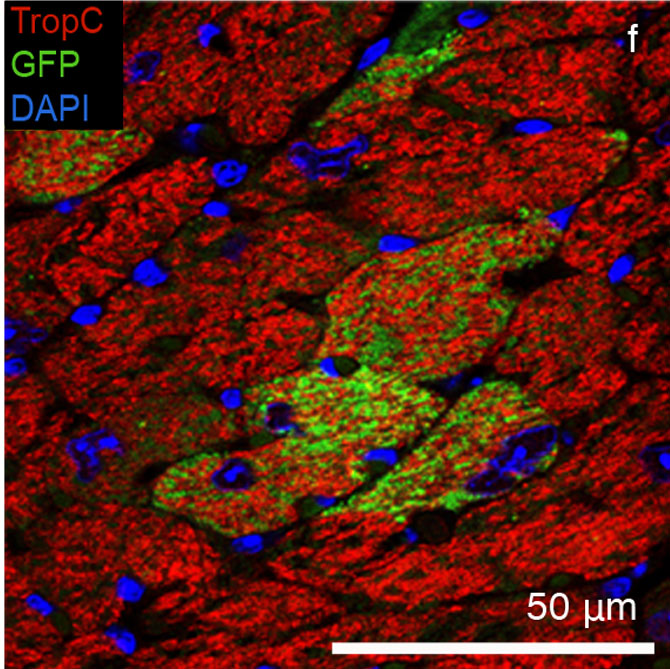
But how do the developed CPCs behave in living organisms? Can these cells really integrate into tissue and regenerate heart muscle? To answer these questions, the scientists injected the GFP-labeled CPCs into the hearts of living mice. After 28 days, the researchers analyzed the hearts and saw that the green fluorescent cells had developed into beating heart muscle cells and had fully integrated into the myocardial tissue of the mouse.
Enormous potential for heart research
Researchers have long tried to stimulate the regeneration of heart muscle cells. For this purpose, they inject stem cells or stem cell-derived cardiomyocytes into the heart. Although the majority of studies found a slight improvement in heart function, in most cases, neither long-term integration nor the differentiation of the cells into heart muscle has been demonstrated.
The result of the group from Schenke-Layland, Nsair and MacLellan provides the first opportunity to generate functioning heart muscle cells, which integrate into the heart muscle. "We are currently focusing on research with human iPS cells. If we can show that cardiovascular progenitor cells can be derived from human iPS cells that have the ability to mature into functional heart muscle, we will have discovered a truly therapeudic solution for heart attack patients", hopes the scientist.
Funding
The work of the research group has been funded by the German-American funding from the Federal Ministry of Education and Research (BMBF) and the California Institute for Regenerative Medicine (CIRM), as well as the Fraunhofer-Gesellschaft (Attract Program), the Ministry of Science, Research and the Arts of Baden-Württemberg, and the US National Institutes of Health (NIH).
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB