The production of industrial suited proteins on demand is the great ambition of the Fraunhofer joint project “Biomolecules from the production line” started in 2011 within the strategic process Biotechnology 2020+ of the German Federal Ministry of Education and Research (BMBF).
Cell-free bioproduction with integrated energy supply
Production of biotechnologically relevant proteins
The availability of high-quality, functional biomolecules is a vital foundation of the ability of our modern, developed society to progress. This increases the demand for enzymes, as well as complex peptides, pharma proteins and synthetic molecules for medicine and pharmacy. Peptide-based substances and their production procedures are currently largely developed with the aid of living cells or organisms. Although this technology is now very efficient, there are significant restrictions on numerous levels. For example, the considerable input of materials and energy limits economic efficiency; many end products have a toxic effect on the cells or organisms that produce them, and the steps for the purification of target proteins and separation of all cellular components of the organism are often very difficult and costly.
Cell-free protein synthesis on an industrial scale
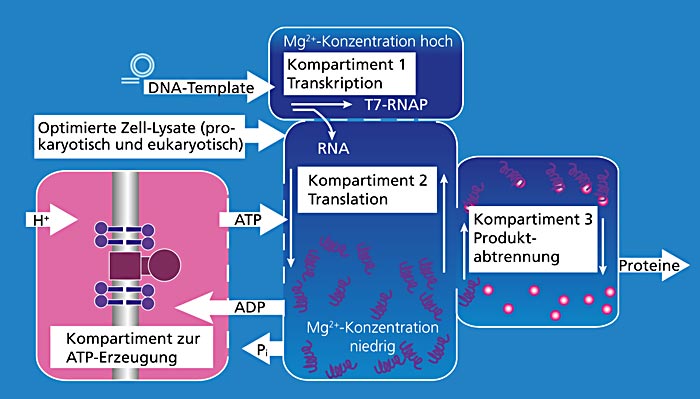
Cell-free protein synthesis provides new opportunities here. The specific application of the components from certain organisms needed for this makes it possible to produce efficiently proteins with complex and even totally new properties in adapted reaction compartments. Despite intensive research in cell-free biosynthesis, we are still currently lacking many basics in order to be able to make economically meaningful use of this technology. This is why eight Fraunhofer institutes, within the BMBF-started the Fraunhofer joint project “Biomolecules from the production line”, launched in 2011 within the strategic process Biotechnology 2020+, are developing and establishing the elements intended to enable the expansion of the technology to an industrial scale. This should involve the transfer of cell-free protein synthesis technology to efficient, compartmented reactor systems.
Energy provision as a limiting factor
The production and supply of energy for the system in the form of ATP (adenosine triphosphate) is of crucial importance. ATP is the main, universal form of energy for all energy-dependent cellular processes and therefore equally important for cell-free biosynthesis. In the cell the highly complex, proton-driven protein complex ATP synthase is largely responsible for the regeneration of ATP. The Fraunhofer IGB is, among other things, concerned with using ATP synthase, in a suitable composition, as an energy generation module for cell-free protein synthesis and thereby set new standards in cell-free bioproduction.
Procedure
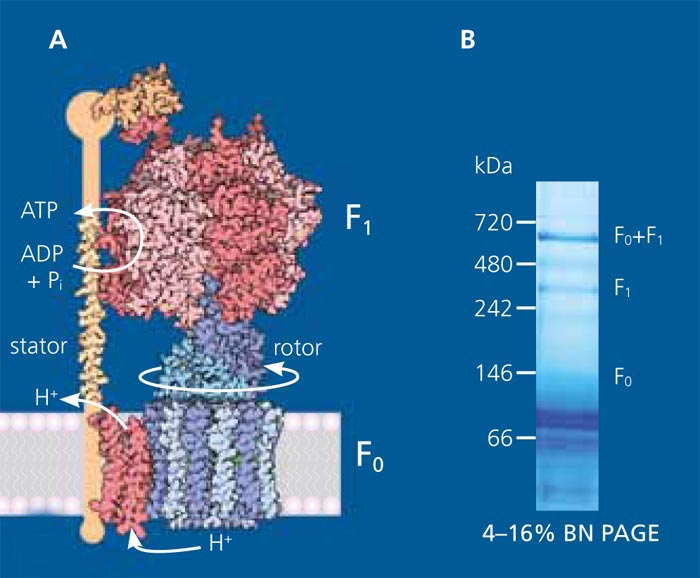
At the Fraunhofer IGB, ATP synthase, a membrane protein complex consisting of eight subunits, is produced and purified in E. coli. The ATP synthase is subsequently directionally and biologically-actively reconstituted in lipid membranes (vesicles and planar membranes). In order to assure ATP synthase the membrane must be energized via a proton gradient. One possibility for this is the use of the protein bacteriorhodopsin from the halotolerant archaebacterium Halobacterium salinarum, which can generate a proton gradient using light. By the co-reconstitution of ATP synthase and bacteriorhodopsin in lipid vesicles, the irradiation of these vesicles with light can regenerate ATP for cell-free biosynthesis.
Components produced in pure form
All components for an energy generation module in pure form were produced at the Fraunhofer IGB:
- In a first approach, the highly complex ATP synthase was functionally isolated in inverted E. coli vesicles. This system previously enabled us to successfully synthesise ATP.
- We also purified ATP synthase by affinity chromatography using a his-tag [2]. This allows the defined and concentrated integration of ATP synthase in lipid vesicles.
- Lipid vesicles with a diameter of 50 nm to 10 μm were produced by membrane extrusion or electroformation.
- We were able to obtain bacteriorhodopsin by osmotic lysis and selective centrifugation from Halobacterium salinarum.
The production of these components in their pure form now enables us to establish the conditions for optimal, stable ATP synthesis.
Outlook
![Diagram of a vesicle for light-driven ATP synthesis (source: [3]). en](/content/igb/en/reference-projects/cell-free-bioproduction/jcr:content/contentPar/sectioncomponent5/sectionParsys/textblockwithpics_4/imageComponent1/image.img.jpg/1627987822316/schema-atp-synthese-700.jpg)
ATP synthase and bacteriorhodopsin are to be directionally co-reconstituted in lipid vesicles or planar lipid membranes. To this end, suitable conditions are to be found in order to be able to continually regenerate ATP over an appropriate period of time. This energy regeneration module is subsequently integrated into a compartment of the cell-free biosynthesis reactor.
Literature
[1] Weber, J. (2006) ATP synthase: subunit-subunit interactions in the stator stalk, Biochim Biophys Acta 1757(9-10): 1162-1170
[2] Ishmukhametov, R. R.; Galkin, M. A. et al. (2005) Ultrafast purification and reconstitution of His-tagged cysteine-less Escherichia coli F1F0 ATP synthase, Biochim Biophys Acta 1706(1-2): 110-116
[3] Choi, H. J. and Montemagno C. D. (2005) Artificial organelle: ATP synthesis from cellular mimetic polymersomes, Nano Lett 5(12): 2538-2542
Projekt partners
- Fraunhofer IBMT, Berlin
- Fraunhofer ISIT, Itzehoe
- Fraunhofer IZM, Berlin
- Fraunhofer IPA, Stuttgart
- Fraunhofer IPK, Berlin
- Fraunhofer IME, Aachen
- Fraunhofer ISI, Karlsruhe
Funding
We would like to thank the German Federal Ministry of Education and Research (BMBF) for their funding of the joint project »Biomolecules from the production line«, within the Biotechnology 2020+ programme and the Fraunhofer-Gesellschaft for funding of the joint project »Basic module for cell-free bioproduction – The industrial cell«, as part of the Fraunhofer system research.
Further information
- Homepage of Fraunhofer researchersHomepage of Fraunhofer researchers (zellfreie-bioproduktion.fraunhofer.de)
- Fraunhofer Lighthouse Project "Cell-free Bioproduction"
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB