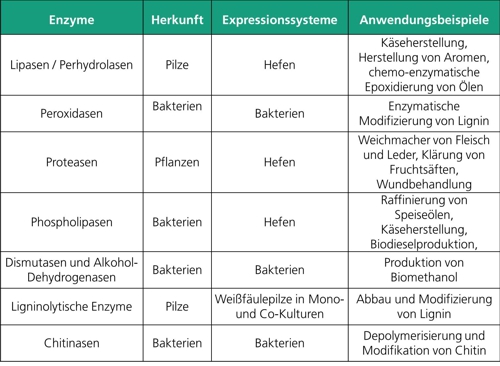
There are various processes for the production of enzymes. One possibility is the direct use of microbial isolates for enzyme production such as white rot fungi for the production of ligninolytic enzymes or chitinolytic bacteria for the production of chitinases. Many of these microorganisms already form the desired enzymes in sufficient quantity under the correct cultivation conditions and without genetic modifications, which can be used directly or after purification in industrial processes.
However, microorganisms often produce not only the desired enzyme, but also a cocktail of different enzymes. In addition, many microbial isolates are not accessible for genetic engineering methods. As a result, modifications of the enzyme to optimize activity, specificity or purification (e.g. by altering the active center or by attaching affinity tags) or to the organism itself that improve enzyme production (e.g. by overexpression of folding aids) can hardly be carried out. In such cases, the desired enzyme can be produced heterologously in established, microbial production strains such as Escherichia coli.
Fraunhofer IGB deals both with the identification of microbial isolates that produce desired enzymes or enzyme mixtures and with the generation of microbial production strains for heterologous enzyme production. Prokaryotic expression systems such as Escherichia coli or Bacillus subtilis and eukaryotic systems such as the yeast Kluyveromyces lactis and the methylotrophic yeast Komagataella pastoris (formerly Pichia pastoris) are used for heterologous enzyme production.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB