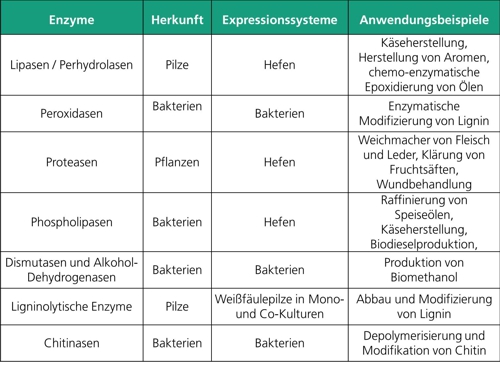
Fraunhofer IGB deals with the production and optimization of enzymes from various classes of enzymes, in particular hydrolytic enzymes such as lipases, phospholipases and proteases, and oxidoreductases such as dismutases, dehydrogenases and peroxidases.
Phospholipase C
Phospholipase C (PLC) catalyzes the hydrolysis of phospholipids to form diacylglycerols and water-soluble phosphorylethanolamine. PLC is used on a large scale for refining vegetable oils in order to achieve a faster and almost complete phase separation.
Lipases
Lipases take up about 5 percent of the world enzyme market due to their versatile application possibilities. Lipases catalyze the hydrolysis of fats (triglycerides) in glycerol and fatty acids. Depending on the reaction conditions, lipases can catalyze further reactions, such as esterification, transesterification, alcohol lysis, acid hydrolysis, aminolysis, acylation, perhydrolysis and epoxidation.
Proteases
Proteases (EC 3.4) are among the most important enzymes in industry. Approximately 60 percent of all commercial enzymes used worldwide are proteases. In industry, proteases are mainly used in pharmaceuticals, medicine and the food industry. Plants offer a broad spectrum of different proteases with different properties. However, in contrast to the production of microbial proteases, the use and production of plant proteases for industry is limited by the often low yields and difficult extraction conditions. Biotechnological processes and the recombinant production of plant enzymes in microbial expression systems, such as yeasts, make large quantities of plant enzymes accessible to industry.
Formaldehyde dismutase
A formaldehyde dismutase from Pseudomonas sp., which has already been successfully investigated for several years from the wild-type strain at Fraunhofer IGB, has a cofactor but also an integrated mechanism for regenerating it, so that this oxidoreductase is well suited for large-scale processes. The substrate formaldehyde and the products methanol and formic acid are important platform chemicals that are processed in large quantities in the chemical industry. Fraunhofer IGB is currently working on the heterologous expression of this enzyme in Escherichia coli. Another oxidoreductase, methanol dehydrogenase, is of industrial relevance, as only a few alcohol dehydrogenases have significant activity against the platform chemical methanol. The work at Fraunhofer IGB includes the heterologous expression of the enzyme and its immobilization and stabilization for use in industrial processes. Together with formaldehyde dismutase, this enzyme will be used to produce methanol from biogas.
Peroxidases
Peroxidases, also belonging to oxidoreductases, are investigated at Fraunhofer IGB with regard to their ligninolytic activities. In addition to peroxidases secreted by white rot fungi to degrade lignin, Fraunhofer IGB is working on new bacterial peroxidases and their role in the lignin degradation process. Bacterial dyp-type peroxidases are a separate class of haem peroxidases and are found in fungi and bacteria. Some of these dyp-type peroxidases are thought to be involved in the degradation of lignin. At Fraunhofer IGB, bacterial dyp-type peroxidases are first expressed heterologously on a laboratory scale in Escherichia coli and the enzyme yield is optimized. Furthermore, the enzymes are purified and their catalytic properties are investigated. A later application can be in the area of lignin modification or the oxidation of aromatic substances in waste water treatment.
Chitinases
Chitinases are another class of industrially relevant enzymes produced at Fraunhofer IGB. These are to be used for the monomerization of chitin, whereby a stream of recyclable materials is to be generated from the waste material chitin (see "Production of recyclable materials from waste streams").
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB