Enzymes are already widely used in the food, textile, cleaning and detergent, chemical and pharmaceutical industries. In research, new enzymes relevant to industry are constantly being discovered, scientifically characterized and optimized in terms of their catalytic activity using modern methods. It is estimated that 10,000 enzymes occur in nature. Of these, 3000 are known, but only about 120 enzymes are used industrially [1].
Fraunhofer IGB is therefore working together with partners from research and industry in the "Innozym" joint project with the aim of developing efficient, recombinant expression processes for the production of technical enzymes and of producing and purifying them up to a scale of 10 m3. This goal is to be achieved by developing suitable production strains and by optimising bioprocess technology.
Innozym - Efficient production of industry-relevant enzymes
Process development and optimization
For the production of industrially relevant hydrolases and oxidoreductases, pro- and eukaryotic expression systems are evaluated and new expression strains and vectors are developed. For variants that lead to high yields on a laboratory scale, we pursue process development and optimization up to pilot scale (10 m3). For studies on scaling up, the Fraunhofer IGB and the Fraunhofer Center for Chemical-Biotechnological Processes CBP will have an integrated multifunctional plant at the Leuna chemical site next year, the design and construction of which is also part of the project.
New expression systems
Currently we are working at Fraunhofer IGB with wild-type and protease-deficient strains of Kluyveromyces lactis and Pichia pastoris as eukaryotic expression strains. Kluyveromyces lactis can be cultivated and induced with different sugars such as lactose from dairy residues. With Pichia pastoris a methylotrophic yeast is used. Methanol is cheaper than many conventional culture media and inductors and the strong promoter of alcohol oxidase I enables a product yield of recombinant enzyme of up to 30 percent of the cell protein. Due to their effective secretion pathways, the strains used are particularly suitable for the production of enzymes that are released into the medium. Both the production control and the product processing of the enzymes are thus considerably simplified.
At Fraunhofer IGB we are currently developing production strains for the production of recombinant hydrolases such as phospholipase C and proteases as well as oxidoreductases. In the course of the project, additional production strains for the production of cellulases, xylanases and lipases are to be developed, which will be used in further projects of Fraunhofer IGB and Fraunhofer CBP.
Example Phospholipase C
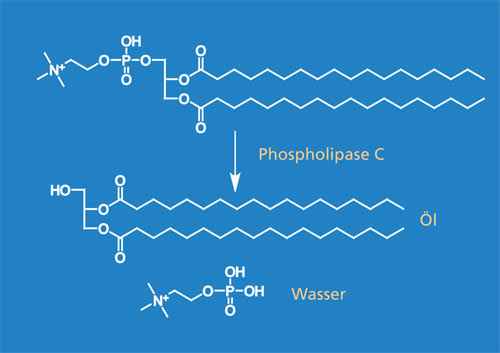
Phospholipase C (PLC) catalyzes the hydrolysis of phospholipids to form diacylglycerols and water-soluble phosphorylethanolamine. PLC is already used on a large scale for the refining of vegetable oils to achieve faster and almost complete phase separation.
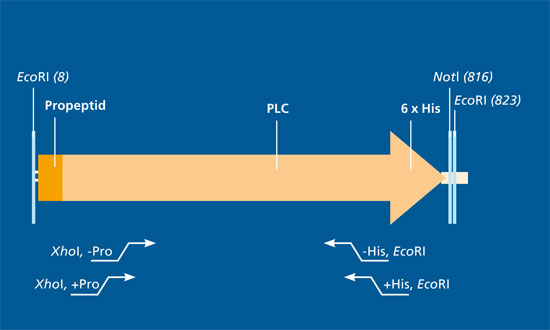
We use expression cassettes with different variants of synthetic structural genes based on the plc sequence of Bacillus cereus. We have also adapted the structural genes to the host organisms used for optimal transcription and translation. The expression cassettes are integrated into the genomic DNA of the expression strains.
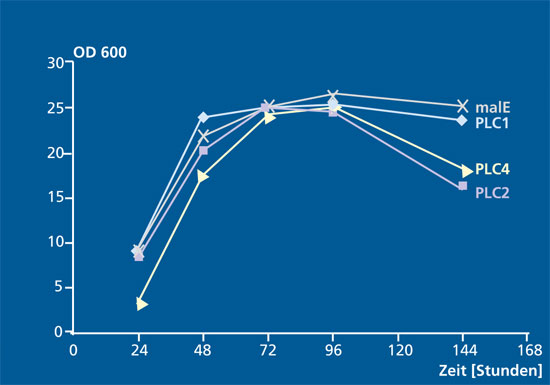
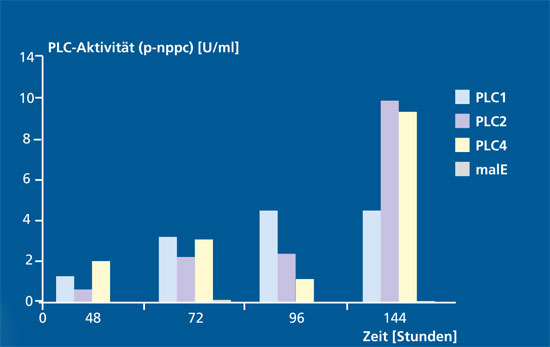
For the expression system Klyveromyces lactis, we have already identified modified strains that carry the corresponding plc construct in multiple copies in the genome. We have investigated the enzyme activity of the recombinant PLC variants against the substrate p-nitrophenylphosphorylcholine using a microtiter plate based activity assay. We could show that the optimized strains secrete PLC in an active form into the culture medium. The quantitative evaluation is the subject of current investigations.
Ligninolytic enzymes from fungi
In the joint project "Innozym", Fraunhofer IGB is working on the identification, characterization and provision of extracellular, ligninolytic enzymes from certain stand fungi, the so-called white rot fungi. Suitable strains and cocultures have already been identified. The expression of lignin cleaving enzymes such as laccases and peroxidases could be optimized by different media composition and inductors. Secreted enzymes are characterised by 2D gel electrophoresis and mass spectrometric detection, chromatographically purified and then biochemically analysed and evaluated. The enzymes are produced in submerged or emergent culture systems and used cell-free for enzymatic lignin digestion, for example in the joint project "Lignocellulose Biorefinery II".
New ligninolytic enzymes from bacteria
Besides white rot fungi, some xylophagous insects are able to use lignified plant material as a food source. This ability of termites and the larvae of some beetle and butterfly species is due to a symbiotic lifestyle with bacteria and fungi. In the joint project "Lignocellulose Biorefinery II" at the Fraunhofer IGB in Stuttgart, the spectrum of available biocatalysts for lignin degradation will be expanded by enriching and isolating symbiontic microorganisms from the digestive tract of larvae of xylophagous insects. In parallel, culture-independent methods (metagenome screening) will be used to identify ligninolytic enzymes of symbiotic origin. In addition, commercially available ligninolytic bacterial strains are tested for their suitability for lignin depolymerization. Suitable enzymes are produced recombinantly and used for cell-free biotechnical processes.
Outlook
Following the molecular biological strain optimization, it is planned to optimize high cell density processes using different carbon sources in fed-batch processes. In addition, we want to evaluate induction strategies with the aim of achieving maximum space-time yield at the Fraunhofer IGB in Stuttgart and up to pilot scale at the Fraunhofer CBP in Leuna. At the same time, downstream processing methods are to be developed, focusing on separation techniques for biomass separation as well as crystallization and chromatography methods for product purification and concentration.
Literature
[1] Braun, M. et al. (2006) Übersichtsstudie Biokatalyse in der Industriellen Produktion, herausgegeben vom VDI
Project information
Project title
Innozym - Efficient production of industry-relevant enzymes
Project duration
March 2009 – June 2013
Project partner
- Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Stuttgart
- University of Stuttgart, Institute for Interfacial Engineering (IGVT), Stuttgart
- c-LEcta, Leipzig
- InfraLeuna GmbH, Leuna
- Linde KCA, Dresden
Promotion
We would like to thank the Federal Ministry of Education and Research (BMBF) for funding the project "Development of innovative processes for the efficient production of enzymes (Innozym)", grant no. AZ 0315510.

 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB