With the expansion of renewable energy generation in the course of the energy transition, surplus electricity is increasingly available depending on the weather, for which various storage and utilization models are being developed. One approach is to use the surplus electricity directly for the production of chemicals. The synthesis of chemicals with renewable energy is not only economical, but also represents – in addition to electricity storage – a sensible utilization path to compensate for an excess supply of electricity. In this way, the chemical and energy sectors will increasingly merge in the future.
Power-to-X and and technologies for the material use of CO2
Our offer: Power-to-X processes for chemical CO2 recycling
The ready availability of renewable electrical energy means that the chemical and energy sectors will increasingly merge in the future as the redox equivalents needed for the synthesis based on CO2 as feedstock can directly be utilized in power-to-X processes.
From catalysts and electrode materials to multi-step synthesis processes
Developments on electrochemical catalysts, electrode materials, ionomer membranes and finally entire systems are taking place at the institute, as well as the coupling of these technologies with additional synthesis processes.
Power-to-X-to-Y: from CO2 to C1 intermediates to complex C compounds
In this way, complex molecular structures can be generated elegantly from the electrochemically produced C1-derivatives methanol, formic acid or formaldehyde using biotechnological processes. This power-to-X-to-Y concept, defined at the institute, has already been successfully demonstrated in the EU project Celbicon or within the ongoing Fraunhofer-internal lighthouse project ShapID.
Electrochemical conversion: power-to-chemicals

For the sustainable electrosynthesis of basic chemicals with electricity from renewable energies, we develop catalysts and suitable electrodes, electrolysis processes and apparatus.
In the Fraunhofer lighthouse project "Electricity as a Raw Material", for example, Fraunhofer IGB has developed a one-stage process that can produce ethene electrochemically in just one process step.
In the EU project Celbicon, we use electrocatalysis to produce methanol from CO2, which is used as a substrate for fermentation.
An electrolysis cell, in which hydrogen peroxide can be produced from water and air using only electrical energy, is already available as a prototype at the IGB.
Competencies
Projects
- Fraunhofer Lighthouse Project "ShaPID"
- CO₂EXIDE – CO₂-based electrosynthesis of ethylene oxide
- ELPEDES – Electrolytic production of H2O2 as the core of a plant for the decentralized, self-sufficient production of disinfectants for hospitals' own use
- E-CO2Met – Electricity & CO2 to Methanol (cbp.fraunhofer.de)
- Fraunhofer Lighthouse Project "Electricity as a Raw Material"
Press releases
Our approach for synthesizing more complex chemicals: Power-to-X-to-Y
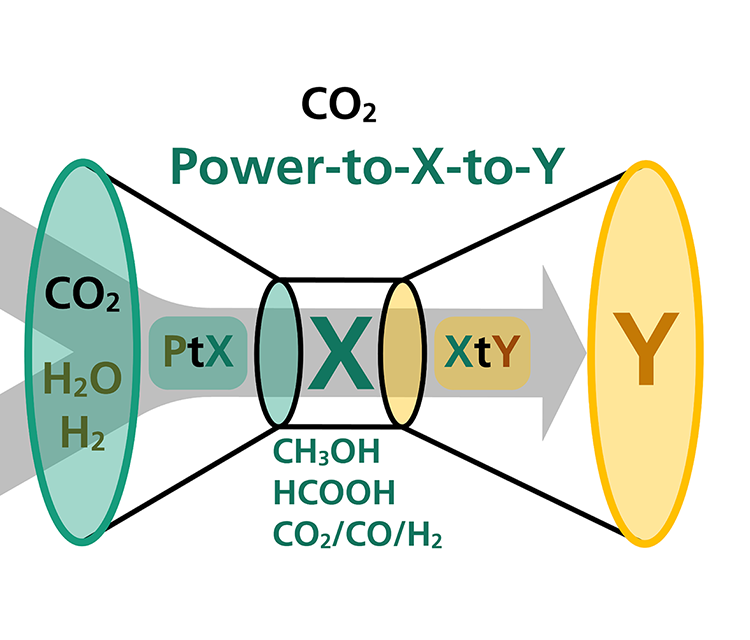
Fraunhofer IGB is extending the power-to-X approach to the power-to-X-to-Y concept, in which the intermediates synthesized from CO2 are further processed in coupled chemical and biotechnological processes to more complex and higher-value products. These processes can be used to develop synthesis routes for a wide range of high value-added chemical products, based on the use of CO2 as a primary feedstock. In this way, the much needed path to an era of independence from fossil resources is being paved.
In the EU project Celbicon, for example, we have produced methanol from CO2 by electrocatalysis, which is used as a substrate for fermentation.
Further information
- Making Money Using Carbon Dioxide?
Projects
Press releases
Chemical use of green H2 at the Fraunhofer Hydrogen Lab Leuna
In partnership with Fraunhofer CBP, the green hydrogen produced at the Hydrogen Lab Leuna can be used on-site for the sustainable synthesis of chemical feedstocks and fuels.
Fraunhofer Hydrogen Lab Leuna
At the Fraunhofer Hydrogen Lab on the Leuna chemical site, technologies for generating green hydrogen are combined with an excellent infrastructure of gas pipelines and gas storage facilities.
Further information
Projects
- Hy2Chem – Construction of a scaling platform for the synthesis of basic chemicals from green hydrogen (cbp.fraunhofer.de)
- E-CO2Met – Electricity & CO2 to Methanol (cbp.fraunhofer.de)
- SynLink – Synthetic e-fuels as key enabler for sector linking (cbp.fraunhofer.de)
Press releases
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB